Know in one minute about ATP synthaseATP synthase is an enzyme that catalyzed ATP synthesis.
|
Introduction
- ATP synthase is an enzyme.
- Found in the mitochondrial membrane of the chloroplast.
- Produces ATP molecules during oxidative phosphorylation (the last stage of cellular respiration).
- ATP is produced in the mitochondrial membrane from ADP and inorganic Phosphate.
- Another name of this enzyme is F1Fo ATPase and complex V.
- Enzymes are substances that act as a catalyst in living organisms.
- ATP synthase catalyzes ATP synthesis in mitochondrial membranes, which are the main energy source of cells for conducting other activities.
ATP synthase location in cells
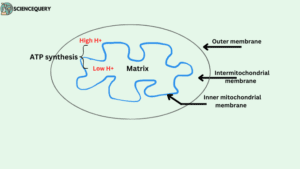
- In eukaryotes, ATP synthase is present in the inner mitochondrial membrane.
- Whereas, in plants, it is found in the thylakoid membrane of Chloroplast.
- In bacterial cells, it is found in the inner surface of the plasma membrane.
What is ATP synthase?
- It is an enzyme that helps in producing ATP.
- Is a mushroom-shaped protein complex.
- It also hydrolyzes the ATP to ADP and Pi.
- For ATP synthesis, enzymes used energy from the movement of protons that generates a proton gradient.
- Electrical energy stored in the proton gradient is transduced into the mechanical energy of a rotating stalk. Which is transduced into chemical energy stored in ATP.
- The enzyme ATP synthase acts as a proton-driving motor and is an example of rotary catalysis.
- Thus, ATP synthase is the world’s smallest molecular motor.
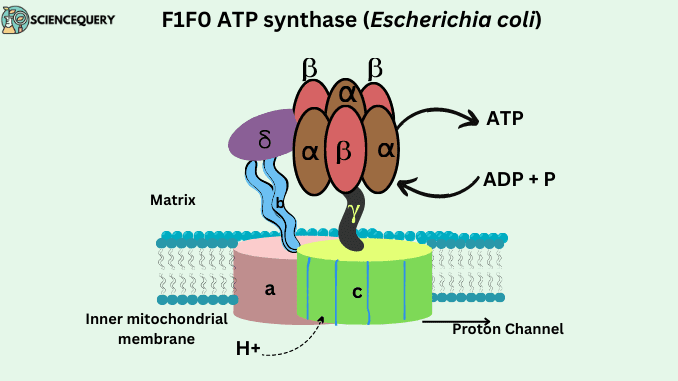
ATP synthase structure and composition
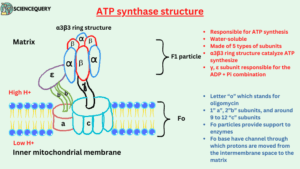
- ATP synthase is a multisubunit transmembrane protein. That is it has so many subunits and is situated between the membrane.
- It is made with F1 and Fo particles. They are the functional units of the ATP synthase enzyme and are responsible for ATP synthesis.
- This enzyme is a lollipop-shaped structure.
- Similar entities have been observed lining the inner surface of the bacterial plasma membrane and in chloroplasts.
F1 particle
- F1 particle is the catalytic site of ATP synthesis because it is responsible for ATP synthesis.
- The F1 region of the ATP synthase enzyme lies in the mitochondrial matrix.
- F1 is a water-soluble peripheral membrane protein, composed of five types of subunits. It is made with 3α (alpha), 1 γ (gamma), 3β (beta), 1δ (delta), and 1 ε (epsilon) subunits. α and β subunits are arranged alternately in the F1 head.
- Three alpha and three β chains combine to form a hexameric α3β3 ring structure that will be responsible for catalyzing the synthesis of ATP.
- γ, ε subunit connect the Fo region to the F1 region. F1 particle is responsible for the ADP + Pi combination.
- If F1 is removed from the urea treatment enzyme cannot synthesize ATP. If, however, F1 is added back to these F0-containing submitochondrial particles, their ability to synthesize ATP is restored.
Fo Particle
- Fo particle is a soluble part of an enzyme.
- Fo particle of ATP synthase enzyme embedded in the inner mitochondrial membrane.
- The “o” in Fo is not a zero but the letter “o” which stands for oligomycin. It is an antibiotic that blocks the Fo particle.
- It consists of 1” a”, 2”b” subunits and there are around 9 to 12 “c” subunits it is majorly made with 2 α (alpha) helices.
- Fo particles provide support to enzymes.
- The Fo base contains a channel through which protons are moved from the intermembrane space to the matrix. Fo provides a channel for H+ ions transportation.
- The F0 subcomplex is composed of channel protein ’C’ subunits to which F1 is attached. F1 -ATP synthase consists of a central gamma (γ) subunit surrounded by alternating α and β subunits (α3 and β3).
Stationary unit
Subunits that are not moved are called stationary units. Subunits “a”, “2b”, “α3” “β3” and delta δ) are examples of stationary units.
Rotor unit
Y, ε, and c subunits are slightly movable so these are called moving units or rotor units.
Mechanism of ATP synthase enzyme
- ATP molecules are synthesized from ADP and Pi with the help of the ATP synthase enzyme. Each subunit of an enzyme play important role in ATP synthesis.
- During ATP synthesis Protons (H+ ions) moved from Fo to F1 for ATP synthesis. γ subunits are very important for proton transportation.
- γ subunit rotates, their proton motive force generate that’s why the proton moved from the F1 particle.
- The γ subunit physically rotates. Its direction is anticlockwise. This induces conformational changes in the β3 subunits that finally lead to the release of ATP. The rotation of the γ subunit allows the interconversion of the β subunit from one state to another.
- The F1 portion of ATP synthase is made with five different polypeptides with a composition of alpha3 β3 hexamer ring. Β subunits act as sites of ATP synthesis. The ring is responsible for:-
- Binding the ADP and inorganic phosphate
- Catalyzing the ATP synthesis.
- Releasing the ATP molecules.
According to the binding change mechanism, the three β subunits of F1 particles have different conformations. One subunit has open (O) conformation, the second has loose (L) conformation while the third one has tight (T) conformation
Tight state (T)- The tight state is responsible for the formation of ATP.
Open state (o)- It is responsible for the binding of ADP and Pi and also responsible for the release of ATP molecules.
Loose state (L)- The bound ADP and Pi become trapped and leave.
The β subunit however can bind the ADP and Pi reactants, synthesize the ATP and release them into the matrix.
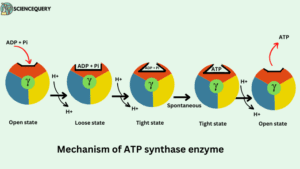
Procedure
- Firstly ADP and Pi are entering the open site.
- The movement of the proton induces an open state to a loose state, in the loose state substrates ADP and Pi are loosely bound.
- Loose state shift to the tight state and substrates affinity increases. ADP and Pi tightly bound and spontaneously condense to form ATP.
- Additional movement of the Proton induces a tight state to again open state for releasing ATP.
- Their affinity for ATP is decreased and the product ATP is released from the catalytic site.
- Once the ATP is released, a new ADP & Pi will enter the open β subunit.
ATP synthase location
In eukaryotes, the ATP synthase enzyme is present in the inner mitochondrial membrane.
The structure of this enzyme is quite elaborate and consists of two major regions the Fo region and the F1 region.
Fo region is embedded in the mitochondrial membrane.
The F1 region lies in the mitochondrial matrix.
The ATP synthase is found in the plasma membrane of bacteria and the thylakoid membrane of Plant chloroplasts. In prokaryotes, this enzyme is present in cytosol because there is no double-bound organelle produced.
ATP synthase complexes in plant
ATP synthase enzyme is a multisubunit enzyme. Its structure contains “αβγ” subunits this complex is called ATP synthase complex.
In the case of plants ATP synthase enzyme is found in the thylakoid membrane of Chloroplast and catalyzed the ATP synthesis processes during the light-dependent reaction.
Chloroplast is a cellular organism found in plants and photosynthetic algae. It contains photosynthetic pigments that produce energy from sunlight.
The machinery for ATP synthesis in a chloroplast is virtually identical to that of a mitochondrion or a plasma membrane of an aerobic bacterium. The ATP synthase consists of a head (called CF 1 in chloroplasts), which contains the Catalytic site of the enzyme, and a base (CFo ), which is present in the membrane and mediates proton movement.
The CF 1 heads lie in the stroma in keeping with the orientation of the proton gradient, which has a higher concentration within the thylakoid lumen. Thus, protons move from higher concentration in the lumen through the CFo base of the ATP synthase and into the stroma, thereby Driving phosphorylation of ADP.
Uses and functions
ATP is a reversible coupling device. That means this enzyme performs a reversible reaction. ADP and inorganic Phosphate bind and produce ATP and on the other hand ATP split into ADP and Pi.
To generate ATP
- ATP is used by cells for the body’s function so ATP is a main source of energy. Energy is stored in our body as a form of ATP.
- This ATP is produced by the ATP synthase enzyme by pi and ADP.
- Each ATP synthase has the ability to produce 100 ATP molecules per second.
- It can also function in reverse to hydrolyze ATP and pump H+ across the inner mitochondrial membrane.
- It catalyzes the condensation of ADP and Pi to form ATP thus the name indicates ATP synthase.
Q&A
What is ATP synthase?
It is a multisubunit enzyme, which produces ATP from ADP and Pi.
Where is ATP synthase located?
In eukaryotes this enzyme is present in the inner membrane of mitochondria and in plants, it is present in the thylakoid membrane of Chloroplast.
Where are ATP synthase complexes located in plant cells?
They are located in plants at the thylakoid membrane of Chloroplast.
Written By: Richa Pachori
References
KARP’s cell and molecular biology, 8th edition (Janet Iwasa & Wallace Marshall)
Biochemistry, 4th edition ( U. Satyanarayana U. Chakrapani)
Lehninger Principles of Biochemistry, 4th edition (David L. Nelson, Michael M. Cox)
