Introduction
The chemical element carbon is one of the most commonly used elements in day-to-day life. One can find carbon in almost all the objects present around. Knowing about its chemical properties, physical properties of objects made by carbon, its atomic structure, etc. is a necessity. By knowing all these properties one can build, construct or design further tools, equipment, and many more objects which are required to complete various tasks, with the help of such objects one can reduce the human effort.
Carbon having atomic number 6 is a nonmetallic element, it has six protons in its nucleus. In the atomic structure of carbon, it consists of electrons orbiting the nucleus in various energy levels or shells.
In the ground state, the carbon has 6 electrons, 6 protons as well as 6 neutrons, giving it an atomic mass of 12. This is defined for carbon 12, while carbon is commonly found in the atomic number 12 there are isotopes of carbon found in the atmosphere as well. For example, carbon-14 has eight neutrons in addition to six protons.
Atomic structure of carbon was proposed and described by various atomic models given by different scientists, physicians, and researchers. Here are a few examples:
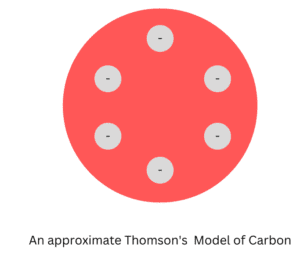
1. Thomson Model of Carbon
In the late 19th century J.J. Thomson gave one of the initial atomic models of an atom, it was known as the Plum Pudding model. According to this model, the atomic structure of carbon would consist of a sphere of positive charge with six electrons distributed evenly throughout it.
2. Rutherford Model of Carbon
The Rutherford Model was proposed by Ernest Rutherford in the early 20th century. He described the atom to be a spherical object. Where a positive charge was concentrated at the center of the atom.
According to this model, the atomic structure of carbon would consist of a small, positively charged nucleus containing six protons and six neutrons, with six electrons orbiting around it.
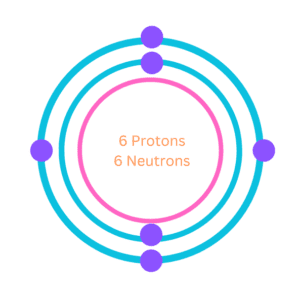
3. Bohr Model of Carbon
Neil Bohr a famous Danish physicist made an important contribution to understanding the structure of an atom and laying the foundation for quantum theory. In the year 1913, he gave Bohr’s atomic model which described the atom as a small, positively charged nucleus with electrons in specific energy levels or shells around it.
According to this model, the atomic structure of carbon would consist of a nucleus containing six protons and six neutrons, with two electrons in the first energy level and four electrons in the second energy level.
 4. Quantum Mechanical Model
4. Quantum Mechanical Model
The Quantum Mechanical Model or the Electron Cloud Model, describes the atom as a small, dense nucleus surrounded by a “cloud” of electrons that move in orbitals or probability distributions around the nucleus.
According to this model, the atomic structure of carbon would consist of a nucleus containing six protons and six neutrons, with electrons in four different orbitals (s, p, d, and f) around the nucleus.
Electronic Model
The structure in which the arrangement of electrons of a Carbon atom is described is known as the electronic model of carbon. The atomic number of carbon is 6 which means that in the ground state carbon has 6 electrons.
The electronic configuration of carbon is 1s22s22p2 . This means that the carbon atom has two electrons in its first shell (1s), two electrons in its second shell (2s), and two electrons in the second shell’s p subshell (2p).
The shells are energy levels on which electrons of an atom revolves, these shells are further divided into subshells. The subshells are used to denote the electronic configuration of any atom. These subshells are written according to the ascending order of their energy values.
The four outermost electrons in the second shell are called valence electrons. These valence electrons are responsible for the chemical behavior of carbon. They are available for bonding with other atoms, and carbon can form up to four covalent bonds by sharing these electrons. This ability to form multiple covalent bonds is what makes carbon such a versatile element and a key building block for many organic molecules.
Overall, the electronic structure of carbon plays a crucial role in determining its chemical properties and behavior. It also explains why carbon can bond with a wide range of other elements to form complex and diverse compounds.
Q&A
1. Carbon’s atomic structure?
Carbon’s atomic structure consists of 6 electrons (in the ground state), 6 protons, and 6 neutrons. The 6 electrons revolve around the nucleus in different shells and subshells.
2. How many carbon atoms are in the structure?
The carbon atom depending upon the type of material has various formations and number of atoms involved.
In diamond, there are 4 carbon atoms bounded with each other while in graphite 3 carbon atoms are bounded with each other.
Summary
Let us now summarise the article and quickly have a look at what we learned from it.
- Carbon is an element that has an atomic number of 6. This means that there are 6 protons in the nucleus of the carbon.
- Different scientists and researchers have introduced the world to various theories of atomic structure. In this J.J. Thomson, Ernest Rutherford and Neil Bohr are some popular names.
- The electronic configuration of the carbon atom is 1s22s22p2 . This means that 2 electrons revolve in the s subshell of the 1st shell while, the other 2 revolve in the s subshell of the 2nd shell. The remaining two are in the p subshell of the 2nd shell.
