
Know in one minute about Immunoglobulins
|
Introduction
Immunoglobulins which are also said to be antibodies are Y Shaped Molecules produced by the plasma cells of the immune system in reaction to the invasion of foreign substances such as bacteria, viruses, or pathogens in the body.
Historical trends in the discovery of Immunoglobulin
- In the early 1900s, active immune components in blood serum were characterised, showing that a certain soluble component could neutralise toxins, precipitate toxins, and agglutinate bacteria.
- This component was initially named based on its activity, such as antitoxin, precipitin, and agglutinin.
- In the 1930s, Elvin Kabat depicted that a fraction of serum called gamma globulin (which is now known as an immunoglobulin) was responsible for these activities.
- These soluble active molecules which are present in the immunoglobulin fraction of serum are currently stated as antibodies.
- The term “humoral immunity” was used to describe the immunologic events involving antibodies because they were found in body fluids known as body humours at that time.
- Antiserum, which is the fraction of serum containing antibodies obtained from individuals exposed to pathogens, such as horses, was given to patients with diphtheria and tetanus.
- The concept of passive immunity originated from these early observations, and today there are still therapies that rely on the transfer of immunoglobulins to protect susceptible individuals.
For example, immune serum containing antibodies against snake or scorpion venoms is commonly used to treat bite victims. This form of immune protection transferred between individuals is called passive immunity because the recipient does not produce their own immune response against the pathogen.
Types of Immunoglobulins and their functions
There are various types of immunoglobulins, also known as antibody classes or isotypes, which are labelled as IgA, IgD, IgE, IgG, and IgM.
Immunoglobulin A, or IgA
Is a protein that is largely present in bodily fluids such as saliva, tears, nasal secretions, digestive system secretions, colostrum, and breast milk. It is the predominant kind of antibody engaged in mucosal immunity and plays a vital part in defending mucosal surfaces against infections.
Immunoglobulin D, or IgD
Mostly found on the surface of mature B lymphocytes but is also present in minute amounts in the blood.
Although its exact purpose is unknown, it is believed to have a role in the activation of B cells and the control of the immunological response.
Immunoglobulin E, or IgE
Is implicated in allergic reactions and serves a function in protecting against parasitic infections.
It is linked to the basophils’ and mast cells’ surfaces, which are implicated in allergic reactions, and is present in the blood in very small amounts.
Immunoglobulin G, or IgG
IgG is the type of immunoglobulin that is found in the blood in the highest concentration and is used to combat bacterial and viral illnesses.
It is also in charge of the long-term immunity that develops after a vaccination or infection. It can cross the placenta and give infants passive immunity.
Immunoglobulin M, or IgM
Is the first type of antibody produced in response to an infection and is normally detected in the blood in its pentameric form.
It participates in the early phases of the immune response, neutralises infections, and effectively activates other immune system parts.
Structure of Immunoglobulin
In 1972, Sir Rodney Porter and Gerald Edelman were granted the Nobel Prize in Physiology and Medicine in recognition of their research that unveiled the structure of immunoglobulins.
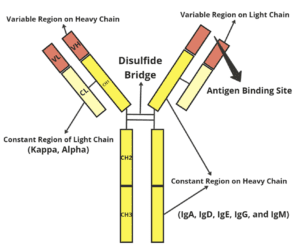
- The IgG antibody is composed of four polypeptide chains, including two light chains and two heavy chains, forming a flexible Y-shaped structure.
- Each chain has a variable (V) region at its amino terminus that forms an antigen-binding site, and a constant (C) region that determines the immunoglobulin
- The functional properties of the antibody are determined by the isotype of the heavy chain.
- The light chains are attached to the heavy chains through disulfide bonds and noncovalent interactions.
- V regions of the heavy and light chains combine to form two identical antigen-binding sites at the tip point of the Y arms.
- Having two antigen-binding sites allows antibody molecules to cross-link antigens and is able to bind to them in a more stable manner.
- The stem of the immunoglobulin structure, also called as Fc fragment, is made up of carboxy-terminal domains of the heavy chains, and it is connected to the arms of the antibody structure by flexible hinge regions.
- Fc fragment and hinge regions may vary in antibodies of different isotypes, determining their functional properties, but the overall organisation of the domains is similar across all isotypes.
Immunoglobulin Class |
Abbreviation |
Location |
Function |
| Immunoglobulin A | IgA | Body secretions (tears, saliva, respiratory and digestive tract, breast milk, etc.) | Protects mucosal surfaces, defends against pathogens in body secretions |
| Immunoglobulin D | IgD | The surface of mature B cells | Function not fully understood; thought to be involved in B cell activation and immune regulation |
| Immunoglobulin E | IgE | Blood; bound to mast cells and basophils | Involved in allergic reactions, defence against parasitic infections |
| Immunoglobulin G | IgG | Blood, tissues | Main type of antibody in the blood, fights bacterial and viral infections, provides long-term immunity, crosses the placenta for passive immunity |
| Immunoglobulin M | IgM | Blood | The first antibody produced in response to infection, activates the complement system, effective in neutralising pathogens |
Q&A
1. Where antibodies are found?
Immunoglobulins are found in blood and other tissues as well as body secretions such as tears, saliva, respiratory and digestive tract creations, breast milk, etc. IgD is found on the Surface of mature B cells.
2. Where antibodies are produced?
Antibodies are produced by B cells also known as Plasma cells.
3. Which immunoglobulins cross the placenta?
IgG is a class of antibodies that can cross the placenta. It does so to strengthen the immune system of the foetus.
4. How are antibodies produced?
- Antibodies are created by B cells in reaction to foreign substances known as antigens.
- Antigen presentation, T cell and B cell activation, plasma cell differentiation, antibody production, antibody release, and memory cell development are the components of the immune response.
- Plasma cells create antibodies, which circulate in the body and bind to antigens to mark them for elimination or neutralisation.
- Long-lasting immunity is provided by memory cells, which “remember” the antigen to facilitate future quicker responses.
5. Are immunoglobulins antibodies?
Yes, they are the same. Antibodies are also known as immunoglobulins.
6. How do antibodies work?
Antibodies work by recognizing, binding to, and neutralising antigens through mechanisms such as neutralisation, opsonization, complement activation, antibody-dependent cellular cytotoxicity (ADCC), and modulation of the immune response.
Written By: Deva Singh
References
- Owen, J. A., Punt, J., Kuby, J., & Stranford, S. A. (2013). Kuby Immunology. W. H. Freeman.
- Paul, W. E. (2008). Fundamental Immunology. lippincott williams & wilkins.
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The structure of a typical antibody molecule.
