Introduction
Purine and pyrimidine are the two nitrogenous bases known as the building blocks of nucleic acids in both DNA and RNA. Besides being nitrogenous bases there are some differences between them so we will discuss purines vs pyrimidines.
Nucleic acid is basically a polymer of countless nucleotides. Nucleotides consist of three subunit molecules, such as a nitrogenous base (also known as nucleobase), a 5-carbon sugar or pentose sugar, and a phosphate group consisting of one to three phosphates. The most important nitrogenous bases in nucleotide formation are purine and pyrimidine (4).
These Nitrogenous bases are organic compounds in heterocyclic form, rich in nitrogen. Also, other elements present in purines and pyrimidines are carbon, hydrogen, and oxygen. The two bases of nucleotides can be single-ringed or double-ringed.
Purines are large and have double-ringed. But pyrimidines have a single ring. Here is a discussion of the importance, functions, and properties of purines vs pyrimidines (3) & (5).
What is purine?
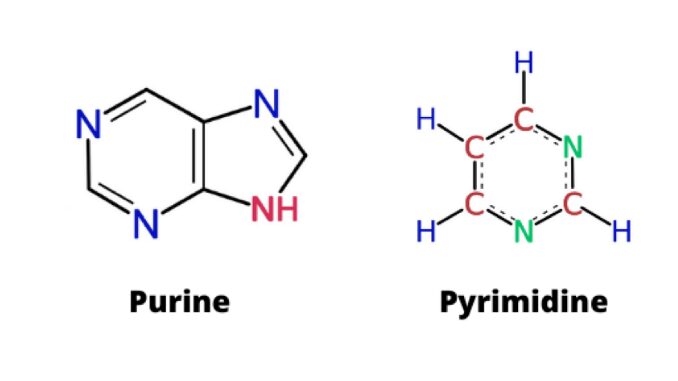
Purines are heterocyclic organic compounds that have a six-membered ring with two nitrogen atoms attached to an imidazole ring. These are the most abundant nitrogenous heterocyclic rings in nature. It is a kind of heterocyclic aromatic chemical compound. The chemical symbol is C₅H₄N₄. Adenine and guanine are the purines found in DNA and RNA. It is also produced naturally in the body (1) & (3).
Source of purine
Every cell in the human body contains purine. There is purine in human food. The amount of purine in human food is generally divided into three parts.
1. The high amount of purine
Meat soup, liver, brain, kidney, fish eggs, yeast, duck meat, beans, cauliflower, cabbage, etc.
2. Middle amount of purine
Chicken, fish, mushrooms, dried beans, lentils, peas, cauliflower, etc.
3. Low amount of purine
Fruits, vegetables, sugar, molasses, honey, milk, egg whites, oils, rice, bread, etc. (2)
Properties of purine
1. It is the building block of DNA and RNA.
2. In addition to the formation of nucleic acids, purine also forms important biomolecules in cells such as ATP, GDP, GTP, NAD, AMP, etc.
3. Purines are synthesized as nucleosides, which are attached to ribose sugars.
4. It has a high melting and boiling point.
5. Uric acid is produced in the catabolism of purine (2) & (4).
What is pyrimidine?
Like benzene, pyrimidine is a single-ringed base. Nucleic acids contain three types of pyrimidine bases, thymine, cytosine, and uracil. Its chemical symbol is C₄H₄N₂. Cytosine and thymine are two nucleobases found in DNA and uracil are found in RNA. Yeasts are excellent sources of pyrimidine (3).
Properties of pyrimidine
1. It is a heterocyclic aromatic compound.
2. Pyrimidine has a six-membered heterocyclic ring with two nitrogen atoms.
3. It has a low melting and boiling point.
4. Pyrimidines are smaller in size.
5. It is insoluble in water.
6. Carbon dioxide, beta-amino acids, and ammonia are produced in pyrimidine’s catabolism (1) & (2).
Discovery
Discovery of purine
The term purine was discovered by German scientist Emil Fischer in 1884. The starting compound of this reaction was uric acid, which was discovered by Carl Wilhelm Scheele in 1776. It was isolated from kidney stones. PCI₅ reacts with uric acid to obtain 2,6,8-dichloropurine, which converts to HI and PH₄I to produce 2,6-aminopurine (1) & (2).
Discovery of pyrimidine
In 1885, Pinner first discovered the name pyrimidine. Pyrimidine was generated including the nucleotides cytosine, thymine, and uracil. It is also found in barbiturates and HIV drugs. Studies about pyrimidine were begun in 1884 (2).
Importance
Purine
It is a very important nitrogenous base.
1. This nitrogenous base is the building block of DNA and RNA.
2. It is important for essential cellular constituents that are involved in energy transfer.
3. Purines also play a vital role in metabolic regulation.
4. It is important for the production of protein and starch.
5. This base is necessary for cell signaling (3) & (5).
Pyrimidine
The importance of pyrimidines are
1. This nitrogenous base also has considerable chemical and pharmacological utility such as antibiotics, antibacterial, cardiovascular, etc.
2. It serves as a form of energy for cells.
4. Pyrimidines are another component of proteins and starches.
5. The two nitrogenous bases of pyrimidine that makeup DNA are thymine and cytosine and the uracil helps in the formation of RNA (1) & (2).
From the above discussion, it is clear that both pyrimidines and purines are very important. Purines and pyrimidines are both fundamental units of the genetic code.
Functions of purines
1. Purines provide energy to the human body.
2. It controls the growth of the cell.
3. The function of purines is the donation of phosphate groups in phosphorylation reactions.
4. It acts as a metabolic signal.
5. This subunit of nucleotides is the primary component of coenzymes, like FAD, NADH, and NADPH, etc.
6. It produced RNA and DNA, proteins, and starches.
7. Purine regulates enzymes in the body.
8. This nitrogenous base generates uric acid in the body. It passes into the cells through the digestion of food. Uric acid is formed when the purines in the cell break down. This uric acid enters the bloodstream directly.
9. Purines can act directly as neurotransmitters (2).
Function of pyrimidines
1. Pyrimidine contributes to the health of any animal by producing amino acids and proteins.
2. It helps in providing nutrition.
3. This base increases the immunity system of the body.
4. It combines with purines to act as the genetic substances in all organisms.
5. Activation of sugars for polysaccharide and phospholipid synthesis is caused by pyrimidine.
6. In pyrimidine synthesis, it forms at various stages which begin with the formation of carbamoyl phosphate.
7. It is the main nitrogenous base present in DNA and RNA. Pyrimidine takes part in the synthesis of DNA and RNA.
8. Pyrimidine also serves as energy for the cell.
9. It is necessary for cell signaling.
10. All enzymes are regulated by pyrimidines (1) & (3).
Purines vs Pyrimidine
The differences between purine and pyrimidine are
Content |
Purine |
Pyrimidine |
Definition |
Purine is a heterocyclic organic compound that has a six-membered ring with two nitrogen atoms attached to an imidazole ring. | Pyrimidine is a single-ringed base and heterocyclic aromatic organic compound. |
Shape |
The shape of purine is larger than pyrimidine. | Its shape is smaller than purine. |
Melting point |
Purine’s melting point is much higher than that of pyrimidine. Its melting point is 214°C. | It has a low melting point. The pyrimidine melting point is 20°C. |
Nucleobase |
It has two nucleobases. These are adenine and guanine. | The three nucleobases of pyrimidine are cytosine, thymine, and uracil. |
Ring structure |
Purine has a double-ring structure. | Pyrimidines have a single six-membered ring structure. |
Boiling point |
It has a high boiling point. The boiling point of purine is 424°C. | Its boiling point is lower than that of pyrimidine. The boiling point of this nitrogenous base is 123°C |
Structure |
These bases have a 9-membered double ring with four nitrogen and five carbon atoms. | Pyrimidine has a 6-membraned single ring with two nitrogen and four carbon atoms. |
Molar mass |
Its molar mass is higher than pyrimidine. The molar mass of purine is 120.11 gm/mol. | The molar mass of pyrimidine is lower than purine. its molar mass is 80.088 gm/mol |
By-products |
The substance produced in the catabolism of purines is uric acid. | The end products in the catabolism of pyrimidine are beta-amino acids, carbon dioxide, and ammonia. |
Synthesis in lab |
Purines are synthesized by tuber purine synthesis. | It is synthesized by the biginelli reaction. |
Soluble |
It is soluble in water. | It is insoluble in water. |
Location of biosynthesis |
The biosynthesis of purines takes place in the liver. | Different tissues are the primary place of biosynthesis of pyrimidine (2) & (4). |
Similarities
In addition to the above differences, some similarities can be observed between purines and pyrimidines. These are-
1. Both purine and pyrimidine are nitrogenous bases.
2. They are the building blocks of DNA and RNA.
3. Both are important for essential cellular constituents that are involved in energy transfer.
4. These two nitrogenous bases are heterocyclic organic compounds.
5. Both have ring structures (1) & (2).
Q&A
1. How to remember purines vs pyrimidines?
- Purines are 9-membraned double-ringed structures. And pyrimidines are 6-membraned single-ringed structures.
- Purines have a double-ringed structure, but pyrimidines have a single-ringed structure.
- Adenine and guanine are the nitrogenous bases of purines. On the other hand, thymine, cytosine, and uracil are the nucleobases of pyrimidine.
In this way to remember purines vs pyrimidines.
2. What can you conclude when you compare the amounts of purines vs. pyrimidines?
The nucleobases of purine in DNA and RNA strands are guanine (G) and adenine (A). Pyrimidines have 3 nitrogenous bases. These are cytosine, thymine, and uracil.
Pyrimidines in DNA are cytosine and thymine and in RNA are cytosine and uracil.
The amounts of purines are larger than that of the amounts of pyrimidine because purines have double rings but pyrimidines have single rings.
3. How many hydrogen bonds are there in purines vs pyrimidines?
Purines always bond with pyrimidines by hydrogen bonds according to Chargaff’s rule. There are two nitrogenous bases present in purines. These are adenine (A) and guanine (G).
On the other side, Pyrimidines also have 3 nitrogenous bases. These are thymine (T), cytosine (C), and uracil (U).
Adenine is bound to thymine by two hydrogen bonds. Guanine bonds to cytosine (in the case of deoxyribonucleic acid) or to uracil (in the case of ribonucleic acid) by three hydrogen bonds. So there are 5 hydrogen bonds in purines vs pyrimidines.
4. How many rings do purines vs pyrimidines?
Purines have a double-ring structure, and pyrimidines have a single-ring structure.
5. Know purines vs. pyrimidines?
- Purines are larger than pyrimidine.
- Pyrimidines have a single-ring structure. But Purines have a double-ring structure.
- Purines’ melting point is higher than that of pyrimidine. Purine’s melting point is 214°C, but pyrimidine’s melting point is 20°C.
- Pyrimidine has 3 nucleobases, such as thymine, cytosine, and uracil. Whereas purines have 2 nucleobases, viz. guanine and adenine.
6. How does this cause complementary base pairing?
Complementary base pairing is the process where guanine is always bound to cytosine with hydrogen bonds and adenine always binds to thymine in DNA. The bond between guanine and cytosine has three hydrogen bonds. And adenine bonds to thymine by two hydrogen bonds.
Purines and pyrimidines are the nucleobases that DNA and RNA strand together through hydrogen bonds. Based on Chargaff’s rule both purine and pyrimidine together through complementary pairing.
Written By: Manisha Bharati
Reference
1. L. Dutta. Inorganic Chemistry: Chemical Elements and their Compounds. Part- II. The New Book Stall, Kolkata. Chapter: Chemistry of Nucleic Acids. Page No: 256 to 271 & Chapter: Biosynthesis of Nucleic Acids. Page No: 565 to 570.
