Introduction
About 40 types of basic elements are needed to sustain life on Earth and among these, there are 6 elements that lay a vital role in sustaining the life of an organism. These 6 elements are carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur. The most essential elements are carbon, oxygen, and nitrogen, and play a significant role in the formation of cells in the body, and in the regulation of various physiological activities. These elements are continuously used and released in the environment thus forming a biogeochemical cycle. The biogeochemical cycle is an essential cycle for all life on earth. Through the biogeochemical cycle, all the elements enter the organism’s body from the natural environment and return to the natural environment from the organisms. One such cyclic process is the nitrogen cycle which is discussed as below (2) & (3).
Nitrogen cycle
The nitrogen cycle is one of the most important biogeochemical cycles as it is the most essential element for all organisms. In this cycle, nitrogen is converted in various ways through biological and physical processes.
Nitrogen is included as an important component of the earth as protein and DNA. The first nitrogen cycle was discussed in 1913. The nitrogen present in the atmosphere rotates from the atmosphere to the biosphere and again from the biosphere to the atmosphere to form the nitrogen cycle. This cycle is reflected in a variety of chemical processes which are as follows (1) & (3).
- Nitrogen fixation
- Ammonification
- Nitrification
- Denitrification
Definition of the nitrogen cycle
The cyclic process by which nitrogen in the atmosphere is transmitted from air to soil, from soil to plants, from plants to animals, and finally from organisms to the atmosphere and maintains the balance of nitrogen in the environment is called the nitrogen cycle (3).
Sources of nitrogen
Nitrogen gas in the atmosphere (79 %) is the main source of nitrogen for all organisms. This gas exists in the atmosphere of the physical environment as gaseous nitrogen (N₂). Other sources of nitrogen are
- Nitrogen is found in the form of nitrates (NO₃) in the soil of the lithosphere and in the water of the oceans in the hydrosphere.
- Nitrogen is present in the form of amino acid that forms proteins in the living environment.
- This element is found in the form of organic molecules such as ribonucleic acid and deoxyribonucleic acid (4) & (5).
The nitrogen cycle in detail
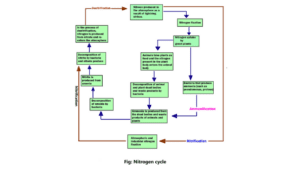 The nitrogen cycle is completed in several stages.
The nitrogen cycle is completed in several stages.
1. Nitrogen fixation
Nitrogen is absorbed by plants only when it is converted to ammonia and nitrate. The process of conversion of gaseous nitrogen to ammonium and nitrate is called nitrogen fixation. Nitrogen fixation is achieved in two ways in nature (1).
A. Physical process
The interaction of nitrogen, hydrogen, and oxygen in the atmosphere requires a lot of energy. The main source of this energy is cosmic radiation, the inherent energy of meteorites, lightning, etc.
During lightning strikes in the sky, atmospheric nitrogen combines with oxygen to form nitric oxide (NO). This nitric oxide is again oxidized by oxygen and converted to nitrogen peroxide. The nitrogen peroxide reacts with rainwater to form nitrous and nitric acid and enters the soil. These two types of acids combine with different mineral salts in the soil to form nitrate compounds (calcium nitrate and potassium nitrate) (3).
The process of nitrogen fixation in the physical method is shown below.
- N₂ + O₂ → 2NO (nitric oxide)
- 2NO + O₂ → 2NO₂ (nitrogen peroxide)
- 4NO₂ + 2H₂O + O₂ → 4HNO₃ (nitric acid)
- 2HNO₃ + CaCO₃ (calcium carbonate) → Ca(NO₃)₂ + CO₂ (calcium nitrate)
B. Biological process
Various soil microorganisms have a significant role to play in fixing atmospheric nitrogen in a natural way. Different types of microorganisms participate in this process. Such as-
- Some soil-dwelling nitrogen-fixing bacteria (clostridium, azotobacter, etc.) and blue-green algae (anabaena, nostoc, etc.) absorb nitrogen directly from the atmosphere and form nitrogen compounds in their bodies. After the death of these bacteria and algae, the nitrogen present in their body is released into the soil.
- There is a type of nodule found in the roots of leguminous plants. The Rhizobium bacteria living in these nodules participate in the nitrogen fixation in the atmosphere. After these bacteria absorb nitrogen directly from the atmosphere, some of the nitrogen is supplied to the host plant and the rest forms nitrogen compounds in their bodies. When the plant dies, the bacteria die and the nitrogen present in the body of the bacteria is released into the soil.
Nitrogen-type inorganic fertilizers are also applied to the soil to increase the fertility of the soil. As a result, the amount of nitrogen in the soil increases (3) & (6).
2. Ammonification
The nitrogen (in the form of nitrate) present in the soil is absorbed by the plant and is located in the protoplasm of the plant cell through the synthesis of amino acids and proteins. The nitrogenous organic compounds present in the plant body are then converted in the following ways.
- Protein and nucleic acids of plant cells are stored in the plant body.
- Plant cell proteins are carried in the animal body following the food chain. The ammonia and ammonium salts present in animal waste substances are converted into amino acids and urea.
- Proteins and nucleic acids present in plants and animals are converted into amino acids by the chemical and biochemical processes.
Amino acid and urea present in the carcasses of plants and animals are re-converted into ammonia and ammonium salts by the analysis of various bacteria and fungi. The process of conversion from amino acids to ammonia is called ammonification. Some bacteria like pseudomonas, proteus, etc. play a major role in this process. These are called ammonifying bacteria (2) & (7).
3. Nitrification
The ammonia or ammonium salt produced in the ammonification is converted to nitrate by the action of various bacteria. This process is called nitrification. Nitrification occurs in two steps.
- At first, ammonia or ammonium salts are converted to nitrite (NH₄⁺ → NO₂⁻). A type of bacteria called Nitrosomonas participates in this process.
- In the second step, the produced nitrite is converted to nitrate (NO₂⁻ → NO₃⁻) by the function of a bacteria called Nitrobacter. Nitrate is dissolved in water and absorbed by plants and assimilated into amino acids and proteins through biosynthesis (2) & (5).
4. Denitrification
In this process, nitrate is converted to gaseous nitrogen, nitrous oxide, nitric oxide, etc.
Denitrifying bacteria such as pseudomonas, thiobacillus, micrococcus, etc. participate in this process.
They decompose nitrates to produce gaseous nitrogen or various oxides of nitrogen and return nitrogen to the atmosphere.
Thus the nitrogen cycle is completed. Some amount of nitrate mixes with groundwater or flows into the sea through the river (3) & (4).
Importance of nitrogen cycle
The nitrogen cycle is an ever-moving process. This cycle is completed by the inflow of nitrogen from the physical environment or atmosphere to the biosphere or living environment. Along with the outflow of nitrogen from the biosphere or living environment to the physical environment or atmosphere. So this cycle is very significant for the environment and all the organisms. The importance of the nitrogen cycle are
- Nitrogen is the main component of protein in the body. This major component of protein enters the organism through the nitrogen cycle.
- Different bacteria participate in the nitrogen cycle. All these bacteria help in the decomposition of dead bodies and waste substances of animals and plants. As a result, the environment is clean.
- The nitrogen required for the formation of cells in the organism is transported to the organism through this cycle. As a result, the ecosystem is maintained for centuries.
- The element nitrogen in the nitrogen cycle is one of the primary components of RNA and DNA.
- The protoplasm of a cell cannot be formed without nitrogen. Therefore, nitrogen enters the organism through the nitrogen cycle, and protoplasm is formed.
- Nitrate and nitrites produced in the nitrogen cycle increase soil fertility and nutritional value after mixing with the soil, which is very important for cultivation.
- Bacteria that help to complete the nitrogen cycle absorb atmospheric nitrogen directly and enter the organism’s body (2).
Effect of the environment and humans on the nitrogen cycle
- The nitrogen cycle is an important biogeochemical cycle. Currently, the nitrogen cycle is being affected by humans and the environment. This natural nitrogen cycle is greatly affected by farming methods.
- At present, the cultivation of some cereals like maize has been increased to produce more food. Besides, the grain is produced on the same land more than once a year. These grains absorb a lot of nitrogen from the soil. This results in nitrogen deficiency in the soil. Nitrogen deficiency in the soil needs to be addressed to increase food production.
- The nitrogen deficiency can be filled by cultivating leguminous plants or by applying organic nitrogen fertilizers. But instead of this method, nitrogenous chemical fertilizer (urea) is produced by industrial methods. Unplanned application of these chemical fertilizers results in an increase in the amount of nitrogen thus disrupting the nitrogen cycle.
- In addition, due to the application of nitrogenous chemical fertilizers, large amounts of nitrate are released from the soil into the canals, ponds, and rivers through rainwater, and water pollution occurs. In this way, the nitrogen cycle is disrupted by the environment and human activities (5) & (6).
The nitrogen cycle has a significant impact on the environment and fauna and plays a vital role in the ecosystem. From the above discussion, it is clear that the bacterial effect is present at each stage of the nitrogen cycle. Atmospheric nitrogen is stabilized in the soil by various bacteria and returns to the atmosphere. This cycle maintains the balance of nitrogen in the system of the earth and the biosphere (3).
Written By: Manisha Bharati
